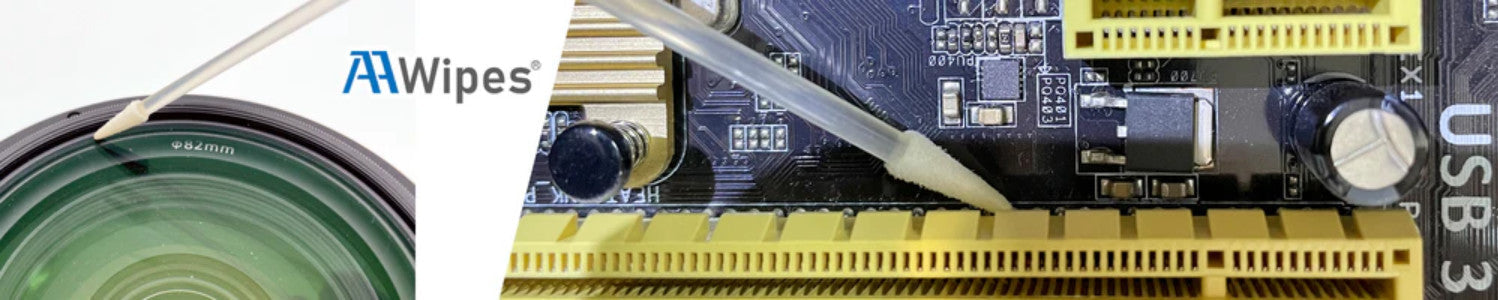
When working in precision cleaning, laboratory testing, or electronics manufacturing, choosing the right swab for a solvent is critical. Incompatible materials can degrade, swell, or shed particles, leading to contamination or equipment damage. This guide explains how different swab head and handle materials respond to common solvents to help you select the most suitable option for your application.
1. What Does Solvent Compatibility Mean?
Solvent compatibility refers to how a swab’s material reacts when in contact with a chemical solvent. Reactions are typically classified as:
-
U – Unaffected: The material maintains integrity and performance.
-
S – Swells: The material softens or expands slightly, possibly affecting precision cleaning.
-
D – Degrades: The material breaks down, dissolves, or becomes unusable.
2. Head Substrate Compatibility Overview
Foam Swabs
Foam heads offer excellent absorption but limited chemical resistance. They tend to swell (S) or degrade (D) when exposed to strong acids (like Chromic or Nitric Acid) or powerful solvents (such as Dimethylformamide).
-
Best performance: Isopropyl Alcohol (IPA), Ethyl Alcohol, and Water.
-
Not recommended: Acetone, strong acids, or chlorinated solvents.
Polyester Swabs
Polyester heads are the most chemically stable and remain unaffected (U) by most solvents, including IPA, Acetone, and Water.
-
Best performance: Ideal for cleanroom environments and precision cleaning with strong solvents.
-
Key benefit: Low lint and high compatibility make them suitable for electronics, optics, and semiconductor applications.
3. Handle Material Compatibility
Polypropylene Handles
Polypropylene offers broad chemical resistance and remains unaffected by alcohols, ketones, and most hydrocarbons. However, it may swell slightly when exposed to certain strong acids or chlorinated solvents.
-
Best performance: Works well with IPA, Acetone, Hexane, and Water.
-
Applications: General-purpose cleaning, electronics, and industrial environments.
Acrylic (ESD-Safe) Handles
Acrylic handles are used where electrostatic discharge (ESD) protection is essential. However, acrylic may degrade (D) under harsh solvents such as Acetone, Benzene, or Toluene.
-
Best performance: Compatible with alcohols and mild cleaning agents.
-
Caution: Avoid use with strong acids or chlorinated hydrocarbons to prevent handle deterioration.
4. Key Takeaways from the Compatibility Chart
| Solvent Type | Best Matched Materials | Avoid Using With |
|---|---|---|
| Alcohols (IPA, Methanol, Ethanol) | Polyester head + Polypropylene handle | None – Excellent stability |
| Strong Acids (Nitric, Sulfuric, Hydrochloric) | Limited options; short contact time only | Foam or Acrylic (ESD) |
| Hydrocarbons (Hexane, Toluene, Xylene) | Polyester or Polypropylene | Acrylic (ESD) |
| Ketones (Acetone, MEK) | Polyester or Polypropylene | Foam or Acrylic (ESD) |
| Water-based Solutions | All materials (Foam, Polyester, Polypropylene) | None |
5. How to Choose the Right Swab for Your Solvent
When selecting a swab for solvent use, consider:
-
Chemical strength – Strong acids or chlorinated solvents require polyester heads.
-
Application sensitivity – Cleanrooms or electronics demand low-lint polyester heads with ESD-safe handles.
-
Frequency of use – Repeated solvent exposure calls for highly stable materials like polyester and polypropylene.
AAWipes offers a complete range of foam, polyester, and ESD-safe swabs designed for cleanrooms, electronics assembly, optics maintenance, and precision manufacturing. Each swab is tested for chemical resistance, lint-free performance, and structural integrity to ensure reliability in demanding environments.